Physicians

The Zenfuze System is a minimally invasive solution for patients suffering from low back pain. Following conservative treatment methods, Zenfuze may be an option. The Zenfuze procedure can treat conditions such as spinal stenosis, spinal instability, and degeneration. Zenfuze may provide patients with a permanent solution for the conditions associated with low back pain. The majority of patients will notice immediate improvement to many or all of their symptoms.
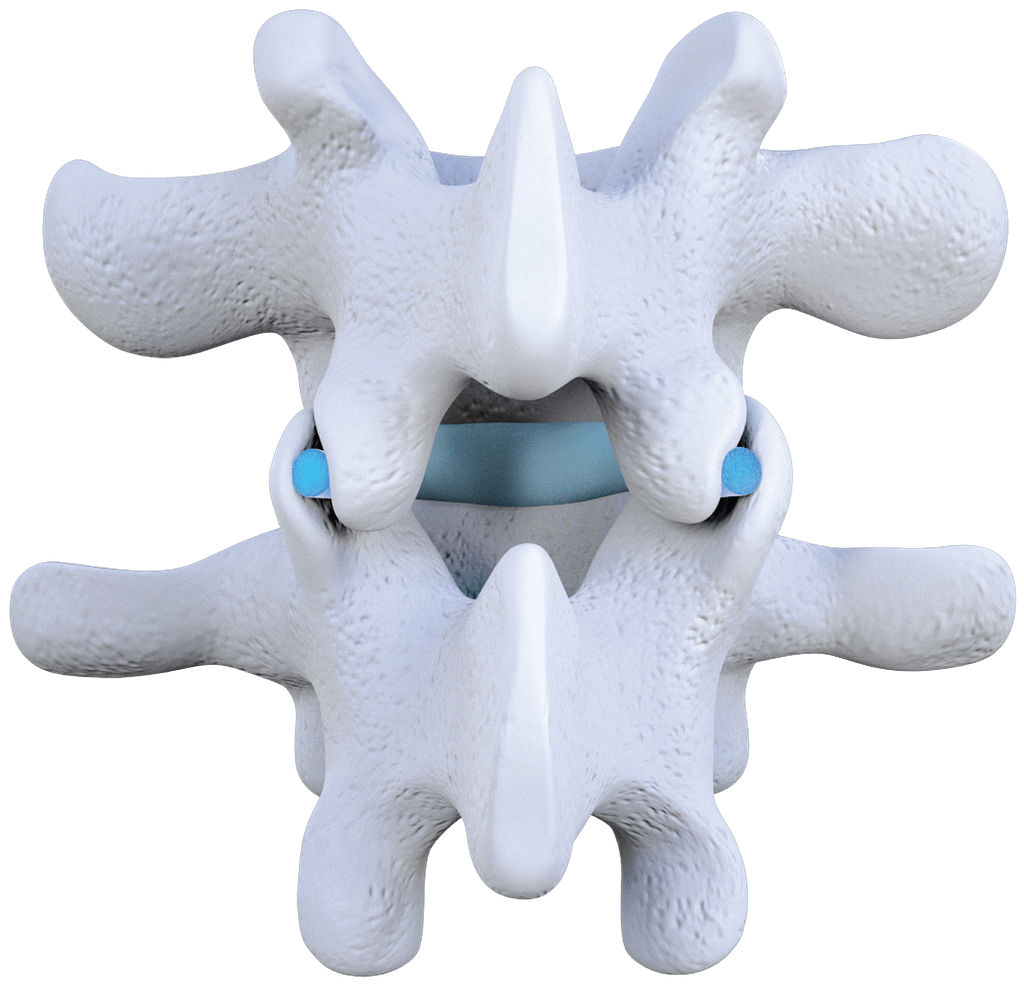

No Metal
The Zenfuze Implants are derived from allograft bone.
Low Complexity
The Zenfuze System is designed to give surgeons fast, easy access to the posterior elements of the lumbar spine.
Safe
The Zenfuze System was developed to prioritize patient safety.
Faster Recovery
Physicians have reported faster patient recovery time compared to instrumented lumbar fusion procedures.
Minimally Invasive
Studies have shown that patients undergoing MIS procedures report less pain and require smaller doses of pain relievers than patients undergoing traditional surgeries.
Excellent Outcomes
Physicians using the Zenfuze System have reported exceptional outcomes.
Zenfuze System
Allograft implants are placed into the lumbar facet joints to re-establish natural joint orientation and separate the joints to reduce inflammation. The Zenfuze System has been designed to stabilize the spinal segment by limiting motion of the joints. Zenfuze still allows for micromotion, which creates an optimal environment for fusion.
Potential Uses
If the patient presents with the conditions listed below, the Zenfuze System may be an option.
- Spinal Stenosis
- Spondylolysthesis
- Instability
- Degenerative Disc Disease
Training

Bioskills
Zentech Spine offers physicians bioskills training and certification for the Zenfuze System.

In-Service
Zentech Spine offers in-service training for physicians and their office staff. A trained Zenfuze representative will give an in-depth presentation on the system.

Proctoring
Zentech Spine offers proctoring by clinicians who are experts in the Zenfuze System.
Need Help?
Frequently Asked Questions
If your patient has tried at least 3 months of conservative, non-surgical, treatment methods (physical therapy, medications, injections) with only temporary pain relief, the Zenfuze System may be an option.
Conservative treatment methods may only offer temporary relief for pain. The Zenfuze System may be a permanent solution for low back pain.
Zenfuze implants are custom milled cortical allograft. Donated human tissue production and processing is regulated by the FDA under CFR Part 1271 – HUMAN CELLS, TISSUES, AND CELLULAR AND TISSUE BASED PRODUCTS. Zenfuze Allograft is processed by licensed tissue banks which are fully compliant with all FDA requirements for tissue processing in the United States.
Zentech Spine and its partners are compliant with regulatory organizations.
- Zentech is a registered tissue bank.
- Zentech is registered with the U.S. Food and Drug Administration (FDA).
- Instruments are manufactured by ISO 13485 certified companies.
- Allograft partners are accredited by AATB and FDA.
- Tumor
- Infection
- Gross Instability
According to physicians currently performing the Zenfuze Procedure, patients may expect to return to Activities of Daily Living (ADLs) within 2 weeks. A back brace will be worn for 6-8 weeks following the procedure. Special attention should be paid to limiting lumbar spine flexion and extension motions and axial rotation. During this period, it is recommended that the patient does not lift over 10 pounds and that they avoid the use of NSAIDs.
Each patient is unique. Specific recovery guidelines will be provided by your physician.

